News
Immune activation during gestation leads to hippocampal neuronal alterations already at birth
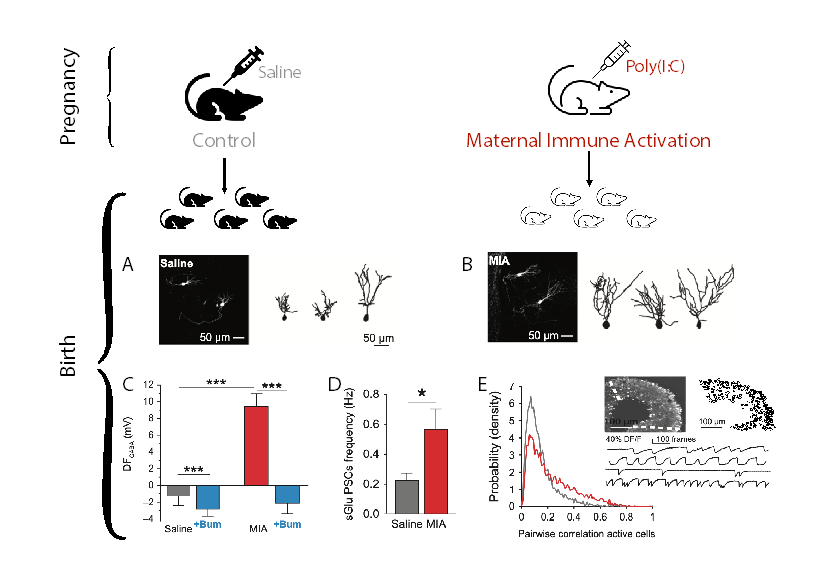
Two of the most biologically complex and vulnerable periods are pregnancy and birth. Birth is a stressful event with a release of several stress molecules needed to facilitate the transition of the fetus to extrauterine life. Furthermore, throughout pregnancy the fetus is susceptible to different types of stressors including immune activation due to maternal infections (maternal immune activation (MIA)). Indeed, bacterial or viral infections during the first or second trimester in humans have been correlated with an increased risk of developing Autism. The in-utero Poly(I:C) mouse model of MIA has been extensively used to investigate the mechanisms leading to the neurological sequels. Using this model, in our recent publication in Cerebral Cortex1 we tested the hypothesis that an in-utero event such as MIA will lead to neuronal alterations already at birth. In our earlier studies2 we described an oxytocin-mediated neuroprotective abrupt GABA polarity shift during birth that is abolished in Autism. We report that in MIA pups at birth the hippocampal network activity is altered and hippocampal neurons have more exuberant apical arbors and dendrites than age-matched controls. Also, MIA abolished the oxytocin-mediated GABAergic inhibitory shift at birth and led to excitatory actions of GABA that persist until at least the 2nd postnatal week. Acute neuronal treatment with Bumetanide at birth restored GABAergic inhibitory action. These observations are reminiscent of those reported in rodent models of Autism and other neurodevelopmental disorders. Our findings both highlight the vulnerability of the critical periods of pregnancy and birth and stress the importance of the GABA developmental shift.
References : 1. Fernandez A, Dumon C, Guimond D, Tyzio R, Bonifazi P, Lozovaya N, Burnashev N, Ferrari DC, Ben-Ari Y. The GABA Developmental Shift Is Abolished by Maternal Immune Activation Already at Birth. Cerebral Cortex. 2019 Aug 14;29(9):3982-3992. 2. Tyzio R. et al. Oxytocin-mediated GABA inhibition during delivery attenuates Autism pathogenesis in Rodent Offspring. Science. 2014, 343 (6171): 675-679. Doi: 10.1126 / science.1247190
Scientific papers
- Smaller brain volumes after birth by Cesarean Section 24 March 2021
- The GABA developmental sequence is altered in a mouse model of Rett Syndrome 26 June 2019
- No stop-growing signal around birth in a rodent model of autism 24 January 2019
- Immune activation during gestation leads to hippocampal neuronal alterations already at birth 4 November 2018
- A promising multicenter trial to alleviate autistic disorders 14 March 2017
- The diuretic Bumetanide and the birth hormone Oxytocin point to a common pathway in the early pathogenesis of Autism in rodents 26 February 2014
- Treating Fragile X syndrome with the diuretic bumetanide: a case report 10 June 2013
- A randomised controlled trial of bumetanide in the treatment of autism in children 11 December 2012
- The GABA excitatory/inhibitory shift in brain maturation and neurological disorders 18 October 2012

