Research
The fundamental research carried out at Neurochlore until 2022 aimed to better understand the construction and formation of the brain during pregnancy and the first years of life, a decisive period for the future of individuals. The aim was also to identify the various alterations that could lead to neurodevelopmental disorders
Neurodevelopment
Brain development (growth of neurons and neural networks, development of brain activity, etc.) begins in utero, is influenced by birth, and progresses during the early postnatal period. ASD, as many other neurodevelopmental disorders, are born in the womb making it instrumental to determine how the initial genetic or environmental insult perturbs brain development leading to disorders.
Neuroarcheology
The concept of Neuroarcheology posits that neurological or psychiatric diseases/syndromes (infantile epilepsies, ASD, mental retardation, genetic diseases, etc.) results from an initial insult which deviates developmental processes. This leads to the presence of neuronal ensembles that do not mature, generate immature activities and are the ultimate cause of the disorders. Therefore, agents capable of selectively blocking the activity of these immature neurons might constitute promising therapeutic agents.

Pregnancy and birth
Pregnancy and birth in mammals, including humans, are biologically complex events and a period of great vulnerability. Indeed, environmental insults occurring during pregnancy increase the incidence of ASD and other neurodevelopmental disorders. During birth, a large amount of stress molecules are released to facilitate the activation of the baby’s respiratory system. Simultaneously, hormones including oxytocin are suggested to protect the brain from the effects of these stress molecules. Our work in rodents is aimed at studying the effects of changes in the time and/or the way of being born (prematurity, birth by cesarean section) on brain development.
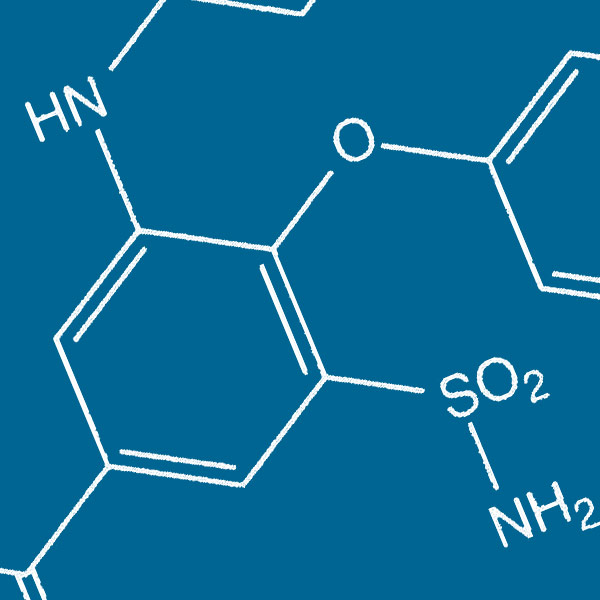
Bumetanide
& GABA polarity
In all animal species and brain structures investigated, there is a progressive shift of the actions of the neurotransmitter GABA: excitation on immature neurons and inhibition on adult ones. This shift is due to a developmentally regulated reduction of intracellular chloride concentrations ([Cl-]i) mediated by an enhanced activity of the KCC2 chloride exporter versus NKCC1, a major chloride importer. This excitatory action of GABA on immature neurons underlies the well-known trophic action of GABA that stimulates cell proliferation and migration as well as neuronal growth and synapse formation. Interestingly, a wide range of pathological insults also lead to high [Cl-]i and excitatory actions of GABA. Our therapeutic efforts are centered on the development of agents like Bumetanide that by inhibiting efficiently the activity of NKCC1 restore GABAergic inhibition.
