Clinical Trials
What’s a clinical trial ?
A clinical trial aims to test the effectiveness and side effects of a new treatment that will be authorized for market release. Clinical trials are preceded by preclinical research phases, including animal models and in vitro approaches, to validate safety, identify potential side effects, and assess effectiveness in reducing the syndrome. This process often takes many years.
What are the phases of a clinical trial ?
Preclinical research:
Before being tested on humans, a potential drug or molecule undergoes laboratory studies, first in vitro and then in vivo on animal models, typically murine models. The aim is to study its effects—efficacy, mechanism of action, and toxicity—at different levels: cellular, target organs, and then at the organism level, including biological and behavioral aspects. If the results are promising, the molecule can proceed to the clinical phase.
Phase I :
Phase I aims to evaluate the safety of a drug by studying its lack of major side effects, its tolerance, and its pharmacokinetic profile (absorption, distribution, metabolism, elimination). It is conducted on a small group of healthy volunteers (20 to 100 participants) in a single-center setting, usually in a single-blind or open-label manner, and lasts for several months. This phase helps identify initial side effects and determine the optimal dose for subsequent studies. The absence of major side effects is crucial for the continuation of the trial.
Phase II :
Phase II, or “pilot study,” aims to evaluate the preliminary efficacy of the drug and fine-tune the dosage while continuing to monitor for side effects. It is conducted on a group of 100 to 300 patients with the targeted condition, randomly divided into two homogeneous and comparable groups (age, sex, disease characteristics, etc.). One group receives the drug under study, while the other receives a reference treatment or a placebo. The trial is conducted in a double-blind manner, meaning that neither the patients nor the medical staff know the nature of the treatment being administered. This phase, lasting from several months to two years, helps confirm the therapeutic benefit and determine the optimal dose for phase III.
Phase III :
La phase III, or “pivotal study,” is the actual comparative efficacy study. It compares the drug to a placebo or an existing treatment in a large number of patients, ranging from 500 to several thousand, in order to confirm its efficacy and safety. Conducted in a randomized double-blind manner, this study takes place across multiple reference centers (multicenter study) over a period of 1 to 5 years. The goal is to demonstrate a positive benefit/risk ratio in a large and heterogeneous population, which is essential for obtaining marketing authorization from the relevant regulatory authorities.
Phase IV :
La phase IV, or “post-marketing,” refers to the long-term monitoring of a drug after it has been authorized for sale. Its goal is to track the drug’s long-term effects, identify any rare side effects or late complications, and assess its effectiveness in real-world conditions. This phase relies on observational studies involving thousands of patients and is part of the pharmacovigilance system, ensuring continuous monitoring of the treatment’s safety.
Neurochlore and its clinical trials
Understanding Autism Spectrum Disorders and the Impact of Fundamental Research
Autism Spectrum Disorders (ASD) encompass a range of complex conditions that affect various aspects of behavior, including social interactions, communication, adaptation to changes, stereotyped behaviors, and restricted interests. These manifestations, which can be severe and pervasive, significantly impact the daily lives and autonomy of those affected, as well as their families and caregivers. To date, no curative treatment exists for ASD.
ASD, like many other neurological conditions, is associated with early brain development abnormalities, often occurring in utero and during the neonatal period. These disorders reflect dysfunctions in the fundamental mechanisms of neurodevelopment, which is a key area of our research at Neurochlore. Our work has focused on the central role of GABA and the abnormalities in the regulation of intracellular chloride, leading to immature neuronal activity, particularly observed in neurodevelopmental disorders.
Bumetanide, a selective inhibitor of the NKCC1 transporter, has emerged as a promising therapeutic candidate to restore this balance by reducing intracellular chloride concentrations, thus enabling GABA to exert its normal inhibitory effect. Based on these principles, we initiated clinical trials to assess the efficacy and tolerability of Bumetanide in patients with ASD, hoping to provide a new therapeutic approach for these neurodevelopmental disorders.
Clinical Evaluation of Bumetanide in Autism Spectrum Disorders:
Our clinical trials involving over 120 children with ASD have explored the efficacy of Bumetanide. The results of these two Phase II studies (Lemonnier et al., 2012; 2017) showed positive effects, strengthening the potential of this molecule as a treatment option. Several other independent studies have confirmed the efficacy of Bumetanide in treating ASD, paving the way for Phase III clinical trials. A meta-analysis (Xiao, Hong-Li, et al. 2024) indicates that over 1,000 children in multiple countries have benefited from this treatment.
Our articles on phases II.a and II.b:
Neurochlore has entered into a licensing partnership with Servier Laboratories to conduct a Phase III clinical trial. This Phase III study, approved by European regulatory authorities, aimed to validate the efficacy of Bumetanide in treating ASD and to prepare for the marketing authorization application in Europe. The study, divided into two age subgroups (2-7 years and 7-18 years), involved 420 children with ASD recruited from 40 centers across Europe, Brazil, Australia, and the United States. Participants were randomly assigned to one of two groups: a Bumetanide-treated group and a placebo group, over a six-month period. Bumetanide was administered in a liquid form, which had been approved and tested in previous Phase II trials.
Details of the phase III studies are available on the following websites:
- For the study of children aged between 2 and 7 years: https://clinicaltrials.gov/ct2/show/NCT03715153
- For the study of children aged from 7 to 18: https://clinicaltrials.gov/ct2/show/NCT03715166

However, the results of this trial did not show a significant difference between the treated group and the placebo group. The treatment efficacy endpoints did not meet the required threshold, thus preventing the validation of Phase III and the market authorization.
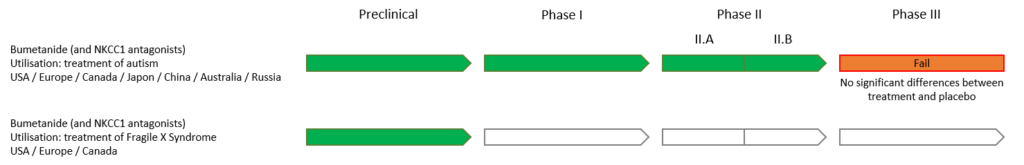
Despite these negative results, the potential of Bumetanide in the treatment of ASD remains an active area of research. Observations from clinicians involved in the study, as well as statistical analyses, suggest that certain subgroups of patients may respond to the treatment. Building on this hypothesis, Neurochlore has invested in a new project aimed at identifying these responders.
Learn more about the projects supported by Neurochlore.

